Selected Team

Company Introduction:
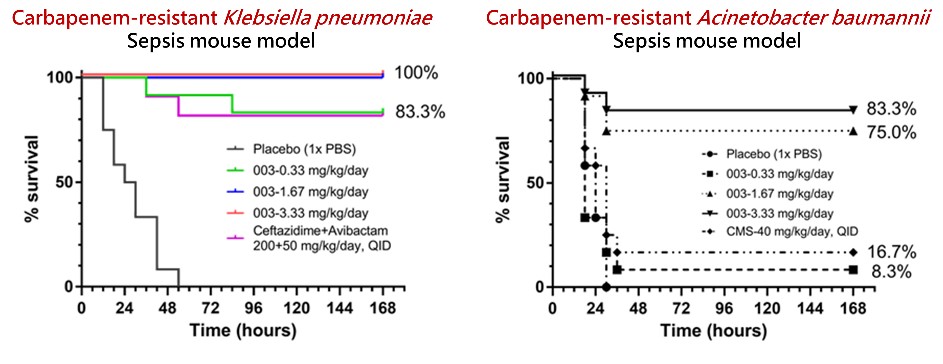
Compared to those on the market (avibactam-ceftazidime, imipenem-relebactam, and others), AS101-003 is a first-in-class antibiotic which could be expected that bacteria would develop resistance slower. The one-step synthesis of AS101-003 economizes the production fee. Organic tellurium compound AS101-003 revealed remarkable activities against Gram-Negative bacterium, including Enterobacterales, Acinetobacter spp., Elizabethkingia spp., and Neisseria gonorrhoeae. The median lethal dose for mice was approximately 23.3 mg/kg. The sepsis mouse models infected by carbapenem-resistant Klebsiella pneumoniae or carbapenem-resistant Acinetobacter baumannii could be rescued using doses of 1.67 and 3.33 mg/kg/day, with survival rates of over 80%.
Core Technology:Anti-infectious agent
Main Products:
Organic tellurium compound, AS101-003, a first-in-class antibiotic
Contact Person:Sung-Pin Tseng
Team Member:Po-Liang Lu
Tsung-Ying Yang

Company Introduction:
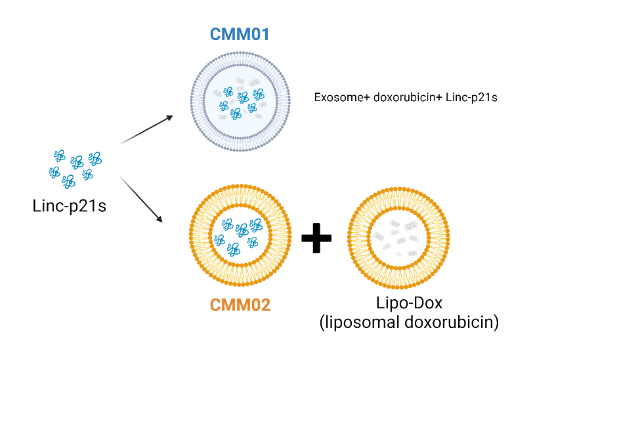
Chemotherapy is pivotal in treating breast cancer, yet only 50% respond favorably. Our solution targets Damaged DNA Binding Protein 2 (DDB2) to combat drug resistance. With support from the National Science Council and SPARK funding, we've completed Proof of Concept. Our CMM01 product, identified through computer simulations, shows promise as a DDB2 inhibitor, enhancing chemotherapy efficacy. Additionally, our second-generation CMM02 product, leveraging lipid carriers by Liposome, aims for early authorization or partial licensing, advancing our goal of IND application and commercialization.
Core Technology:Targeting DDB2 to enhance chemotherapy efficacy and reduce side effects in breast cancer treatment.
Main Products:
RNA-Based Chemosensitizer
Contact Person:Wei-Chien Huang
Team Member:Liu, Liang-Chih
Hsin-Chiao Chou
Yu-Hao He
Ya-Ling Wei
Chih-Hao Huang

Company Profile:
FerroptoCure, Inc. is a biopharmaceutical company committed to the development of ferroptosis-inducing chemotherapy. FerroptoCure was established in May 2022 based on over 15 years of intensive research at Keio Univ..
Company Introduction:
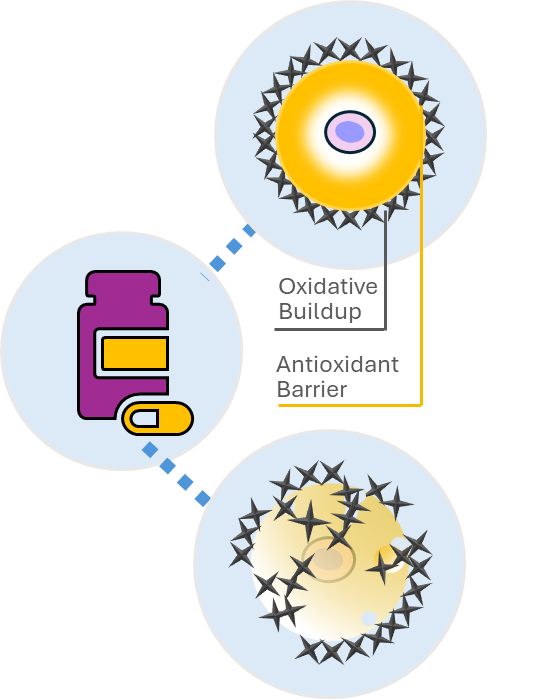
To develop the ferroptosis-inducing anti-cancer therapy, we are focusing two targets that cancer cells use to prevent oxidative stress: xCT and ALDH work together collaboratively to suppress the oxidative stress. Using a synthetic lethality approach, we were able to strongly induce ferroptosis through the simultaneous inhibition of both xCT and ALDH. xCT is also expressed in greater amounts in cancer cells than normal cells, therefore, we can induce ferroptosis specifically in cancer cells, with little damage to normal cells. In our development, we have confirmed a high antitumor effect and safety not only for Triple-Negative Breast Cancer (TNBC) but also for many treatment-resistant solid tumors. Furthermore, our approach involves the use of low molecular weight compounds, which eliminates the need for specialized and costly techniques such as cell therapy or antibody drugs.In our lead candidate (FC-001), we started a Phase I study in Japan in January 2024 for TNBC.
Core Technology:Anticancer drugs that target the antioxidant systems in cancer cells to induce ferroptosis, aiming to overcome treatment resistance.
Main Products:
Small molecule drugs
Contact Person:Yuji Otsuki
Team Member:Hideyuki Saya
Osamu Nagano
Hiroyuki Hanada
Tkahiro Ouchi

Company Introduction:
Our team focuses on the mechanisms of genesis of endoplasmic reticulum (ER) stress in neurodegenerative diseases, which leads to cytotoxicity and neurodegeneration. ER stress-induced cytotoxicity is a central underlying mechanism shared by Huntington’s disease (HD) and all major neurodegenerative diseases, including Alzheimer’s and Parkinson’s diseases and ALS. The team’s research on cellular and mouse models of HD has led to the discovery of a promising therapeutic approach to the disease, boosting the unfolded protein response (UPR). Several cell-protective pathways are activated through the UPR, one initiated at the ER membrane by the PERK kinase.
Core Technology:Developing a small molecule activator of PERK to enhance cellular protective. mechanisms against ER stress-induced cytotoxicity in neurodegenerative diseases.
Main Products:
Therapeutic product

Contact Person:Gerardo Lederkremer
Team Member:Marina Shenkman
Chaitanya Patel
George Atmeh
Doron Eren

Company Introduction:
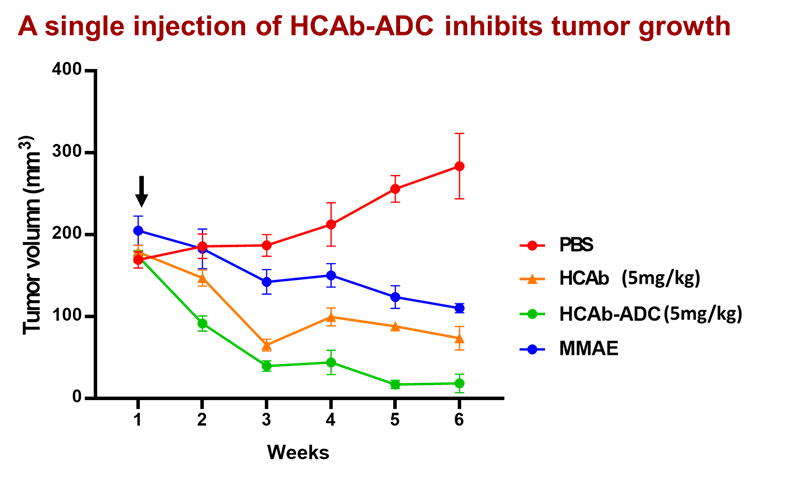
Carcinoembryonic Antigen-Related Cell Adhesion Molecule 6 (CEACAM6) is a phosphatidyl inositol (GPI)-anchored glycoprotein specifically expressed on the surface of cancer cells. We generated a anti-CEACAM6 heavy chain antibody (TMU HCAb) from a camelid immunized library to recognize glycosylated CEACAM6 which possesses characteristics such as small molecular weight, high specificity, high affinity, stability, and strong tissue penetration. We developed TMU HCAb-ADC (Antibody-Drug Conjugate) which can effectively eradicate CRC cells in xenograft models. In addition, HCAb-ADC selectively kills CEACAM6- expressing tumor cells with minimal toxicity to peripheral blood mononuclear cells (PBMC), highlighting its potential as an ADC candidate.
Core Technology:Utilizing a heavy-chain antibody (HCAb) linked to a tubulin inhibitor via hydrophilic linkers to selectively target and kill CEACAM6-expressing epithelial cancer cells.
Main Products:
Antibody-drug conjugate
Contact Person:Ming-Heng Wu
Team Member:Tsai-Mu Cheng
Kuo-Hsiang Chuang
Cheng-Chung Lee
Min-Hsuan Yen
Yao-Tsung Tsai


Company Profile:
In terms of mission, our company specializes in the development of ex vivo tumor microenvironment culture technologies and application solutions. Utilizing our unique culture techniques
and patented designs, our MedSelect series products provide accurate and stable tumor microenvironment simulation and drug screening solutions, assisting clients in the field of
anti-cancer drug development.
In terms of vision, our company aspires for the MedSelect products to become a driving force in reducing the use of experimental animals in the field of tumor science research.
Company Introduction:
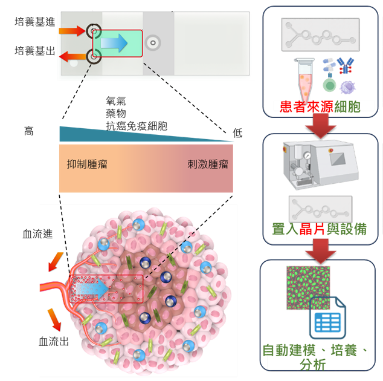
After obtaining tumor samples from patients post-surgery, they are dissociated, stained with apoptosis indicators, and transplanted into tumor microenvironment chips. An automated pumping and analysis system is then employed to assess the efficacy of drugs predicted by physicians.
Core Technology:MedSelect provides fully automated real-time regional drug response analysis
Main Products:
MedSelect is a tumor microenvironment chip and automated machine
Contact Person:Hsuan-Yu Mu
Team Member:Ya-Hui Lin
Jen-Huang Huang

Company Introduction:
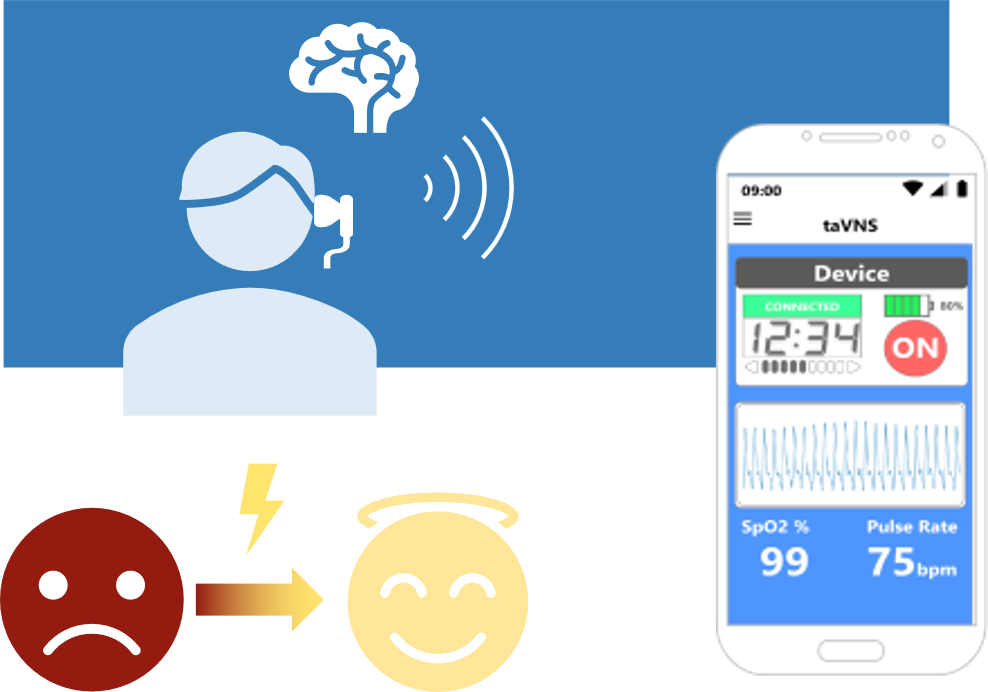
The product development of this project integrates the taVNS control interface and in-ear optical measurement information into a single information platform, inspired by existing neckband-style headphones on the market. It will design taVNS electrode fixation mechanisms and in-ear optical measurement mechanisms that conform to the human ear shape, and conceal power sources and stimulation and measurement circuits within an integrated neckband mechanism. Integrated with wireless transmission technologies such as Bluetooth or WIFI into smart mobile devices, users can control the device through a single interface and obtain real-time optical measurement information.
Core Technology:Transcutaneous auricular vagus nerve stimulation (taVNS) targets vagus nerve branches in the ear, offering potential therapeutic applications for various neurological and psychiatric conditions with minimal side effects.
Main Products:
A wearable taVNS- Relaxi
Contact Person:Jia-Jin Chen
Team Member:Chien-An Chen
Chun-Wei Wu


Company Profile:
We have formed an innovative team by combining cardiovascular surgery experts from National Taiwan University Hospital in Taipei and Hsinchu, the team from the Institute of Electronics at National Yang Ming Chiao Tung University, and the technology industry in the Hsinchu area. We are dedicated to the research and development of continuous testing and monitoring technologies needed to improve the critical care and emergency medical fields since 2021.
Company Introduction:
Based on our research and core technologies, we participated in the NTU SPARK program from August 2021 to July 2023. We revised our prototype products and received multi-disciplinary training in IP applications and the regulation processes of medical devices, some of which are must-know in start-up companies. Our product's Technology-Readiness-Level (TRL) is level 5-6, and we have been receiving another acceleration program for prototype maturation this year. Eventually, this subject from the multi-disciplinary collaboration won a National Innovation Award in December 2023. We demonstrated the functional tests of the prototype on the BMCC-NSTC platform in May 2024.
Core Technology:Electro-Chemical (Non-Enzyme) & Semi-conduct layout & Inkjet printing at FPCB
Main Products:
Real-time Lactate/PH/Temperature Sensor for Extra Corporeal Circuit

Contact Person:Hsiao-En Tsai
Team Member:I-Sshang Chen
Yu-Ting Cheng
Tai-Wei Huang
Jacky Ks Hung
Chia-Peng Shih

Company Introduction:
The new tracheal endoscope with four-way rotation function and inflatable balloon can perform "selective lung ventilation" and can be used in border lung tumor detection and hemostasis.
Core Technology:endobronchial blocker with sensor and a bendable head design to expand the applicability of the endobronchial blocker, allowing flexible adjustment of the angle to the desired position during intubation, and minimizing the risk of surgical interruption.
Main Products:
Auxiliary medical devices

Contact Person:Chun-Yu Wu
Team Member:Pai-Sai Chen
Jo-Ting Chao
Ting-Hsuan Chen
Chia-Yuan Hsieh


Company Profile:
OpenHand Robotics specializes in designing robotic hands for stroke patients, aiming to provide effective rehabilitation training at home. Led by a team of professional occupational therapists, our mission is to empower patients with innovative solutions that enhance their recovery journey. Our cutting-edge technology ensures that patients receive high-quality rehabilitation in the comfort of their own homes, promoting independence and improving quality of life
Company Introduction:
Our product, the iOpen EMG, is a home-use robotic hand specifically designed for stroke patients. Utilizing electromyography (EMG) signals, the iOpen EMG detects the user’s intention to move and assists in opening tightly clenched fingers through its specially designed mechanical structure. This facilitates meaningful rehabilitation training. The device is accompanied by a comprehensive set of rehabilitation programs created by professional occupational therapists. These programs aim to help patients regain independence and dignity as quickly as possible. The iOpen EMG is a groundbreaking tool in stroke rehabilitation, providing patients with the opportunity to perform effective and consistent therapy in the comfort of their own homes.
Core Technology:Utilize electromyography (EMG) signals to detect user intention, enhancing rehabilitation through targeted home-use robotic assistance.
Main Products:
A rehabilitation system

Contact Person:Kai Zhang
Team Member:Jer-Hao Zhang
Wandy Gu
Company Profile:
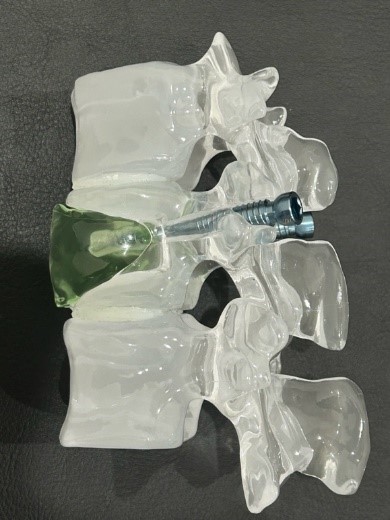
We, Spine Chronicle Japan, are a start-up that challenges the aging spine. Conventional spine surgery implants are not optimal for elderly patients, and surgical results are poor because of weakened bone quality. We are developing new spine surgery implants to solve this social problem.
Company Introduction:
Spine Chronicle Japan have a solution for spine surgery. We are developing spinal implants that improve surgical results in elderly patients with weaker bones. Our implants fortify the spine with minimally invasive surgery, leading to better recovery time.
Core Technology:Spine surgical implants with screws and bone cement.
Main Products:
Spine surgical implants
Contact Person:Ryo Kitagawa
Team Member:Noritaka Yonezawa
Seiji Hirasaki